Here’s a creative yet neutral introduction:
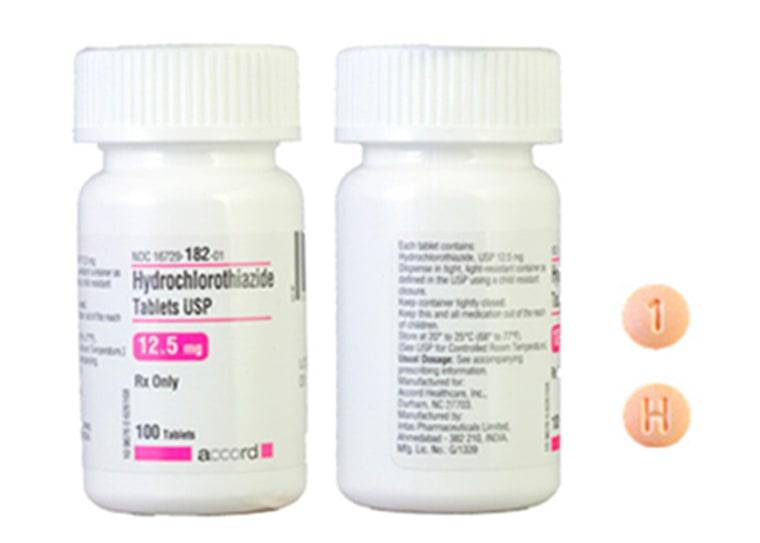
In the quiet corridors of pharmaceutical regulation, a sudden alarm echoes with potential life-threatening consequences. The Food and Drug Administration (FDA) has once again stepped into the critical role of public guardian, issuing a recall that sends ripples of concern through the nation’s healthcare landscape. A blood pressure medication, once trusted by millions, now stands suspended under scrutiny, its invisible contaminants threatening to transform a daily health routine into a potential medical nightmare. As dawn breaks across America, patients and healthcare providers alike find themselves navigating an unexpected terrain of medical uncertainty, where the line between treatment and risk becomes perilously thin. In a critical move that underscores the ongoing challenges in pharmaceutical safety, the Food and Drug Administration (FDA) has initiated a recall of a widely prescribed blood pressure medication due to potential life-threatening contamination risks. The decision comes after extensive internal testing revealed dangerous chemical compounds that could pose significant health hazards to patients.
Manufacturers of the affected medication have been immediately notified, with production and distribution halted to prevent further potential exposure. Healthcare providers across the nation are now scrambling to contact patients currently using the specific drug variant and recommend alternative treatment options.
Preliminary investigations suggest the contamination involves trace amounts of a carcinogenic compound known to increase long-term health risks. While the exact origin of the contamination remains under investigation, initial reports point to potential manufacturing process irregularities at one of the primary production facilities.
Patients currently taking the recalled medication are advised to consult their healthcare providers immediately. Medical professionals emphasize the importance of not abruptly discontinuing blood pressure treatments, recommending careful consultation to establish safe replacement protocols.
The recall affects multiple dosage strengths and batch numbers, highlighting the extensive nature of the potential risk. Pharmaceutical experts suggest this incident underscores the critical need for more rigorous quality control measures within the drug manufacturing industry.
Consumer protection agencies are closely monitoring the situation, with potential legal implications for the responsible pharmaceutical company. Patient safety advocacy groups have already called for more transparent reporting mechanisms and enhanced pre-distribution testing protocols.
Financial markets have responded swiftly, with the pharmaceutical company’s stock experiencing significant downward pressure following the announcement. Investors and industry analysts are closely watching the potential long-term reputation damage and potential legal ramifications.
Health insurance providers are working to expedite coverage for alternative medications, recognizing the urgent need to ensure continuous treatment for patients managing hypertension. Many are waiving typical prescription transfer fees to facilitate seamless medical care.
The FDA’s decisive action reinforces its commitment to protecting public health, demonstrating a proactive approach to identifying and mitigating potential pharmaceutical risks. As investigations continue, patients and healthcare providers remain vigilant, prioritizing safety and comprehensive medical care.
The ongoing situation serves as a critical reminder of the complex challenges inherent in pharmaceutical manufacturing and the continuous need for robust safety protocols.